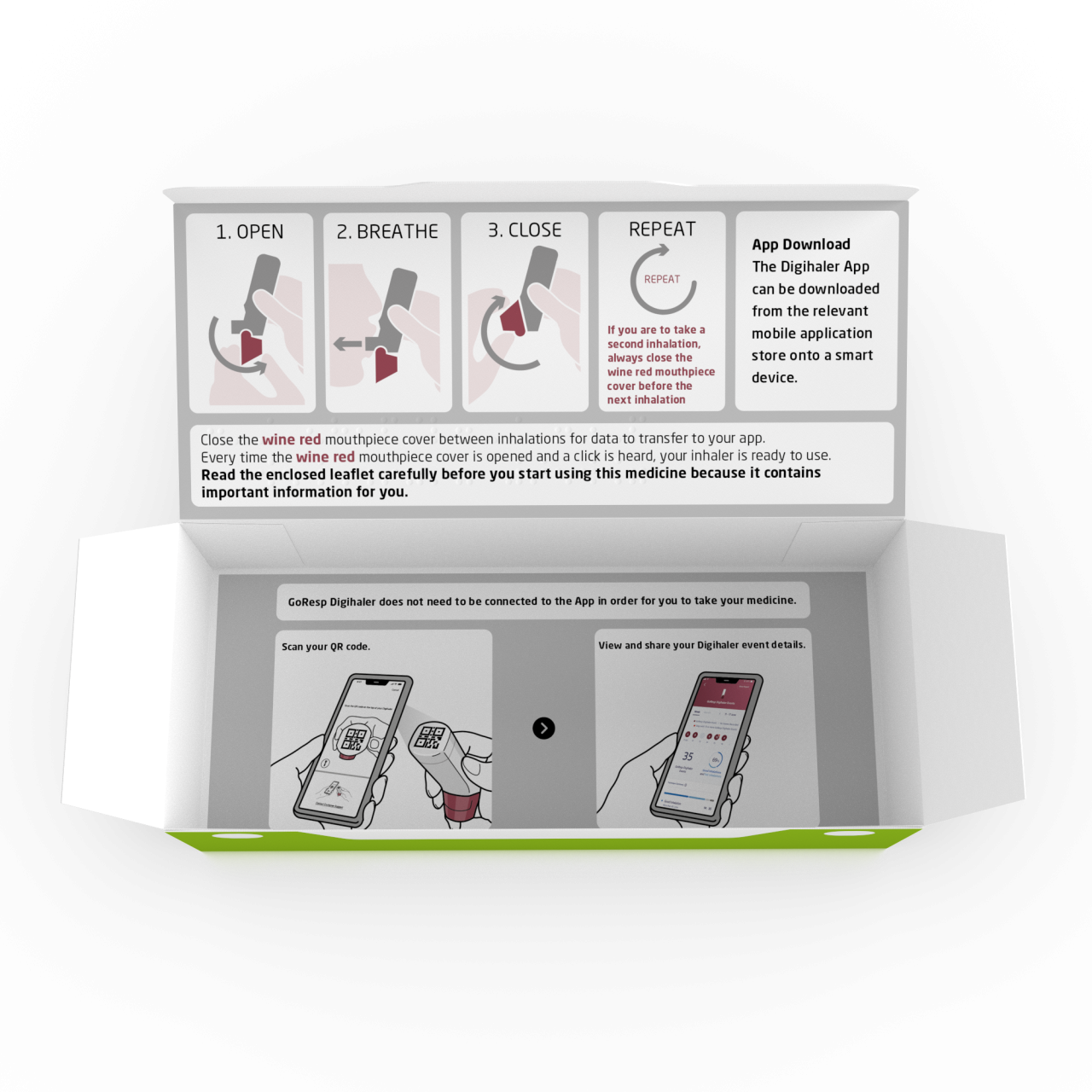
GoResp Digihaler 160micrograms / dose / 4.5micrograms / dose dry powder inhaler (Teva UK Ltd) 180 dose
Medium adult steroid dose
(based on 4 puffs / day)
- Adult Asthma Licence
- COPD Licence
Pathways
The use of each medicine included in Right Breathe has been considered in relation to international, national, and regional prescribing pathways. The pathway points at which this medicine is considered a viable prescribing option are included below. If you wish to view the entire pathway, click on its heading, from where you can also access all the prescribing options for each pathway and each pathway point.
Prescribing
Detailed prescribing information is provided below. This content has not been generated by the RightBreathe team, but has been integrated from a 3rd party solution: Multilex (provided by FirstDataBank). Use of this prescribing content is subject to the FirstDataBank disclaimer, which is set out in the "About" section of RightBreathe.
As RightBreathe is a bespoke decision support tool, it covers each and every individual known inhaler option on the UK market to a high level of specificity. Multilex, as a more general resource, does not offer the same level of specificity. There are therefore a small number of medicines for which there are discrepancies between RightBreathe and Multilex content, most notably in relation to licensed particulars and associated licensed doses.
Given the high level of specificity the RightBreathe team work to, details of the individual inhaler licence are most likely to be summarised accurately in the content provided at the top of this page, rather than in the Multilex content below. Where there is ambiguity, users may also wish to refer to individual summaries of product characteristics prior to prescribing.
Dosing
Chronic obstructive pulmonary disease (FEV1<70% predicted normal)
| Type | Age Range | Dose | Licensed |
|---|---|---|---|
| Maintenance | From 18 years | 2 actuations - TWICE a DAY - inhalation |
Asthma: Maintenance
| Type | Age Range | Dose | Licensed |
|---|---|---|---|
| Maintenance | From 12 years to 18 years | 1 actuation - ONCE a DAY - inhalation - using a metered dose device | |
| Maintenance | From 18 years | 1 actuation - ONCE a DAY - inhalation - using a metered dose device | |
| Maintenance | From 12 years to 18 years | 1 actuation - TWICE a DAY - inhalation - using a metered dose device | |
| Maintenance | From 18 years | 1 actuation - TWICE a DAY - inhalation - using a metered dose device | |
| Maintenance | From 12 years to 18 years | 2 actuations - ONCE a DAY - inhalation - using a metered dose device | |
| Maintenance | From 18 years | 2 actuations - ONCE a DAY - inhalation - using a metered dose device | |
| Maintenance | From 12 years to 18 years | 2 actuations - TWICE a DAY - inhalation - using a metered dose device | |
| Maintenance | From 18 years | 2 actuations - TWICE a DAY - inhalation - using a metered dose device | |
| Maintenance | From 18 years | 3 actuations - TWICE a DAY - inhalation - using a metered dose device | |
| Maintenance | From 18 years | 4 actuations - TWICE a DAY - inhalation - using a metered dose device |
Asthma: Maintenance and reliever therapy
| Type | Age Range | Dose | Licensed |
|---|---|---|---|
| Maintenance | From 12 years to 18 years | 1 actuation - TWICE a DAY - an additional actuation may be taken to relieve symptoms, followed by a further actuation after a few minutes if needed - inhalation - using a metered dose device - Maximum dose 8 actuations DAILY | |
| Maintenance | From 18 years | 1 actuation - TWICE a DAY - an additional actuation may be taken to relieve symptoms, followed by a further actuation after a few minutes if needed - inhalation - using a metered dose device - Maximum dose 8 actuations DAILY | |
| Maintenance | From 12 years to 18 years | 2 actuations - ONCE a DAY - an additional actuation may be taken to relieve symptoms, followed by a further actuation after a few minutes if needed - inhalation - using a metered dose device - Maximum dose 8 actuations DAILY | |
| Maintenance | From 18 years | 2 actuations - ONCE a DAY - an additional actuation may be taken to relieve symptoms, followed by a further actuation after a few minutes if needed - inhalation - using a metered dose device - Maximum dose 8 actuations DAILY | |
| Maintenance | From 12 years to 18 years | 2 actuations - TWICE a DAY - an additional actuation may be taken to relieve symptoms, followed by a further actuation after a few minutes if needed - inhalation - using a metered dose device - Maximum dose 8 actuations DAILY | |
| Maintenance | From 18 years | 2 actuations - TWICE a DAY - an additional actuation may be taken to relieve symptoms, followed by a further actuation after a few minutes if needed - inhalation - using a metered dose device - Maximum dose 8 actuations DAILY |
Asthma: Reliever therapy
| Type | Age Range | Dose | Licensed |
|---|---|---|---|
| Maintenance | From 12 years | 1 actuation - when required - inhalation - using a metered dose device - Maximum dose 8 actuations DAILY |
Safety Advice Warnings
Adverse effects (report/action)
- Corticosteroids may cause growth retardation in children under 18 years
- During transfer from oral steroids allergic conditions may be unmasked
- High doses may affect bone metabolism
- If growth in children is slowed, consider referral to a paediatrician
- If visual disturbances occur, perform ophthalmic evaluation
- May reduce serum potassium levels
- Prolonged or high dose may lead to adrenal suppression
- Systemic effects possible with any inhaled corticosteroid
Advice concerning formulation
- Not all available brands are indicated for all uses
- Not all available brands are licensed for all age groups
Advice on Administration
- Consider use of a spacer device for suitable patients and formulations
Advice on drug withdrawal
- Do not withdraw this drug suddenly
Advice on possible excipients
- Some formulations contain lactose
Discontinue due to test or exam
- Discontinue if paradoxical bronchospasm occurs
Dose changes (other conditions)
- Dose varies with brand
- Maintain treatment at the lowest effective dose
- Not licensed for all indications in all age groups
Patient Counselling
- Advise patient to rinse mouth with water after each dose
- Advise patient to seek medical advice if asthma seems to be worsening
- Advise patient to seek medical advice if treatment is ineffective
- Advise patients to avoid chickenpox, measles etc - see doctor if exposed
- Advise visual disturbances may affect ability to drive or operate machinery
- Consider issuing Steroid Treatment/Steroid Emergency Card
- Ensure patient has a fast acting bronchodilator available
- If reliever required frequently consider alternative without corticosteroid
- Patient should seek medical advice if signs of pulmonary infection develop
- Patients may not taste/feel any medication when using dry powder inhalers
- Use regularly to maintain freedom from symptoms
Pre-treatment points to consider
- Correct electrolyte disorders before treatment
- Therapy should not be initiated during exacerbation of asthma
Recommended monitoring
- Check patient is using correct inhaler technique
- Consider monitoring ECG in patients at risk of QT prolongation
- Monitor adrenal function in stressed patients e.g.surgery/intensive care
- Monitor blood glucose closely in patients with diabetes mellitus
- Monitor for signs/symptoms of pneumonia in patients at risk
- Monitor regularly the height of children receiving prolonged treatment
- Monitor serum electrolytes
- Monitor serum potassium regularly in severe asthmatic or hypoxic patients
- Re-evaluate therapy in active or quiescent pulmonary tuberculosis
Safety/advice in other condition
- Caution in transfer from oral steroids in adrenal insufficiency
- Systemic corticosteroids may be needed during elective surgery
- Systemic corticosteroids may be needed during periods of stress
Drug Interactions
| With | Risk | Severity |
|---|---|---|
| (LEVACETYLMETHADOL) | Hypokalaemia with high dose beta-agonist increases risk of arrhythmias | Significant Risk: Usually avoid combination. Use combination only under special circumstances, taking any necessary action to reduce risk. |
| ATOMOXETINE | May increase risk of cardiac effects | Significant Risk: Usually avoid combination. Use combination only under special circumstances, taking any necessary action to reduce risk. |
| DOMPERIDONE | Predisposing factors may increase risk of arrhythmias | Significant Risk: Usually avoid combination. Use combination only under special circumstances, taking any necessary action to reduce risk. |
| METHACHOLINE | Methacholine effect reduced; review dosing, see product literature | Significant Risk: Usually avoid combination. Use combination only under special circumstances, taking any necessary action to reduce risk. |
| SOTALOL | Effect antagonised/ predisposing factors increase risk of arrhythmias | Significant Risk: Usually avoid combination. Use combination only under special circumstances, taking any necessary action to reduce risk. |
| (ASTEMIZOLE) | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| (HALOFANTRINE) | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| (RETIGABINE) | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| (SODIUM STIBOGLUCONATE) | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| AJMALINE | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| AMIFAMPRIDINE | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| AMIODARONE | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| ANAGRELIDE | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| ARSENIC TRIOXIDE | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| ARTEMETHER AND LUMEFANTRINE | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| AVAPRITINIB | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| AZITHROMYCIN | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| CHLOROQUINE | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| CILOSTAZOL | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| CIPROFLOXACIN | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| CITALOPRAM / ESCITALOPRAM | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| CLARITHROMYCIN | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| COCAINE | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| DELAMANID | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| DISOPYRAMIDE | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| DONEPEZIL | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| DRONEDARONE | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| DROPERIDOL | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| ENTRECTINIB | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| ERYTHROMYCIN | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| ETELCALCETIDE | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| FLECAINIDE | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| FLUCONAZOLE | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| GLASDEGIB | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| HALOPERIDOL | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| HYDROXYCHLOROQUINE | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| HYDROXYZINE | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| IBUTILIDE | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| INOTUZUMAB | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| IVOSIDENIB | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| LEVOFLOXACIN | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| MELPERONE | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| METHADONE | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| MOBOCERTINIB | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| MOXIFLOXACIN | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| ONDANSETRON | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| OSILODROSTAT | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| OSIMERTINIB | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| OXALIPLATIN | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| PAPAVERINE | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| PENTAMIDINE | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| PHENOTHIAZINES (QT 1) | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| PIMOZIDE | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| PIPERAQUINE AND ARTENIMOL | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| PRALSETINIB | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| PROPOFOL | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| QUINIDINE | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| SELPERCATINIB | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| SERTINDOLE | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| SEVOFLURANE | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| SULPIRIDE | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| TERLIPRESSIN | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| VANDETANIB | Predisposing factors may increase risk of arrhythmias | Moderate Risk: Minimise risk. Take action necessary to reduce risk. Counsel patient. |
| ACE INHIBITORS WITH THIAZIDE DIURETICS | Increased risk of hypokalaemia with high doses of beta agonist | Low Risk: No action needed. Risk of adverse outcomes appears small and depends upon the condition of the patient. Counsel patient. |
| ACETAZOLAMIDE | Increased risk of hypokalaemia with high doses of beta agonist | Low Risk: No action needed. Risk of adverse outcomes appears small and depends upon the condition of the patient. Counsel patient. |
| ANGIOTENSIN II INHIBITORS AND THIAZIDE DIURETICS | Increased risk of hypokalaemia with high doses of beta agonist | Low Risk: No action needed. Risk of adverse outcomes appears small and depends upon the condition of the patient. Counsel patient. |
| BETA BLOCKER EYE DROPS | May antagonise effect of each other | Low Risk: No action needed. Risk of adverse outcomes appears small and depends upon the condition of the patient. Counsel patient. |
| BETA BLOCKERS | May antagonise effect of each other | Low Risk: No action needed. Risk of adverse outcomes appears small and depends upon the condition of the patient. Counsel patient. |
| BETA BLOCKERS AND THIAZIDE DIURETICS | May antagonise effect of each other | Low Risk: No action needed. Risk of adverse outcomes appears small and depends upon the condition of the patient. Counsel patient. |
| LOOP DIURETICS | Increased risk of hypokalaemia with high doses of beta agonist | Low Risk: No action needed. Risk of adverse outcomes appears small and depends upon the condition of the patient. Counsel patient. |
| THEOPHYLLINE | Increased risk of hypokalaemia with high doses of beta agonist | Low Risk: No action needed. Risk of adverse outcomes appears small and depends upon the condition of the patient. Counsel patient. |
| THIAZIDE DIURETICS | Increased risk of hypokalaemia with high doses of beta agonist | Low Risk: No action needed. Risk of adverse outcomes appears small and depends upon the condition of the patient. Counsel patient. |
Precautions
- Adrenal insufficiency
- Arterial aneurysm
- Breastfeeding
- Children aged 6 to 18 years
- Diabetes mellitus
- Electrolyte imbalance
- Family history of long QT syndrome
- Galactosaemia
- Glucose-galactose malabsorption syndrome
- Hepatic cirrhosis
- History of torsade de pointes
- Hypertrophic obstructive cardiomyopathy
- Hypokalaemia
- Idiopathic subvalvular aortic stenosis
- Ischaemic heart disease
- Lactose intolerance
- Major risk factors for decreased bone mineral content
- Phaeochromocytoma
- Pregnancy
- Pulmonary tuberculosis
- Severe cardiac failure
- Severe cardiovascular disorder
- Severe hypertension
- Tachyarrhythmia
- Thyrotoxicosis
Contraindications
- Children under 6 years
- Long QT syndrome
- Torsade de pointes
Side Effects
- Adrenal suppression
- Aggression
- Anaphylactic reaction
- Angina
- Angioedema
- Anxiety
- Atrial fibrillation
- Behavioural disturbances
- Blood pressure changes
- Blurred vision
- Bronchospasm (paradoxical)
- Bruising
- Candidiasis (mouth or throat)
- Cardiac arrhythmias
- Cataracts
- Cough
- Cushing's syndrome
- Decrease in bone mineral density
- Depression
- Dermatitis
- Dizziness
- Dysphonia
- Exanthema
- Extrasystoles
- Glaucoma
- Headache
- Hoarseness
- Hyperglycaemia
- Hypersensitivity reactions
- Hypokalaemia
- Increase in blood levels of free fatty acids
- Increase in blood levels of glycerol
- Increase in blood levels of insulin
- Increase in blood levels of ketones
- Muscle cramps
- Nausea
- Palpitations
- Pneumonia
- Prolongation of QT interval
- Pruritus
- Psychomotor hyperactivity
- Sleep disturbances
- Suppression of growth in children and adolescents
- Supraventricular tachycardia
- Tachycardia
- Taste disturbances
- Throat discomfort
- Tremor
- Urticaria
- Visual disturbances